Overview
We take our inspiration from Nature, which utilizes extensive hydrogen-bonding networks within proteins and metalloenzymes to influence structure as well as function. We are interested in controlling the secondary coordination sphere of metal complexes by establishing non-covalent interactions in order to promote the activation of small molecules. We have also prepared porous materials containing immobilized metal complexes, thereby creating an environment around the metal ion that is similar to that in metalloenzymes.
Small Molecule Activation
Hydrogen bonding interactions influence the secondary coordination spheres of metal ions in proteins. To emulate these architectures, synthetic complexes with hydrogen bonding motifs are being developed.
High-Spin Metal–Oxo Complexes
Development of Heterobimetallic Complexes
New Complexes with a Redox Active Framework
C–H Bond Functionalization

Artificial Metalloproteins
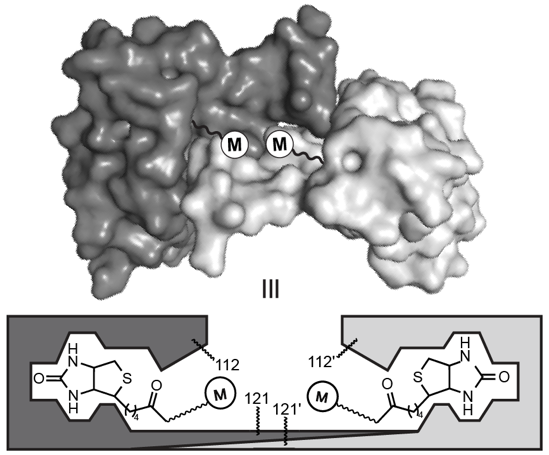
In another method to control the microenvironment surrounding a metal ion, we take a chemogenetic approach. Here, chemical synthesis of biotinylated ligands allows us to anchor the resultant metal complexes into a protein host. Through genetic manipulation of this host, we can systematically modulate the covalent and noncovalent interactions incurred between amino acid side chains and the metal ion(s).
Artificial Blue Copper Proteins
Biologically Relevant Reactive Intermediates
Biomimetic Multinuclear Active Sites